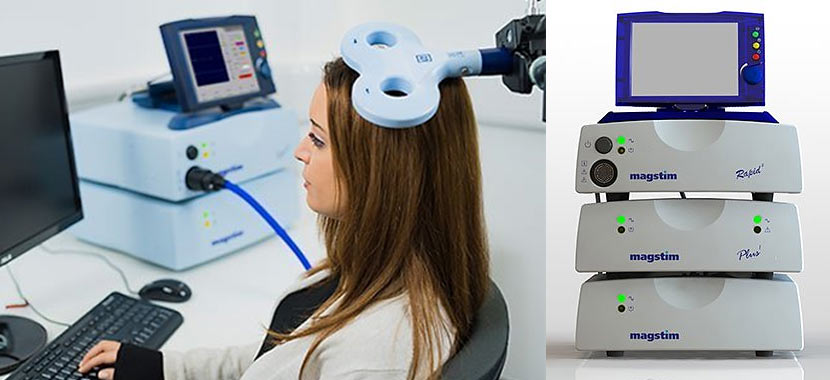

The Magstim Rapid² is capable of high-frequency repetitive protocols for both cortical* and peripheral stimulation. With a package to fit a wide variety of research purposes, the Magstim Rapid² has been used in research studies
worldwide.
The Magstim Rapid² is compatible with the full range of Magstim coils and has the ability to connect an EMG module providing a versatile, effective research tool to fit a variety of budgets and research purposes.
Ideal for rTMS clinical studies and therapeutic investigations.
Users in the USA, The Magstim® Rapid² and Super Rapid² are FDA 510(k) cleared for the stimulation of peripheral nerves. The Magstim® Rapid² Therapy System is FDA 510(k) cleared for the treatment of major depressive disorder in adult patients. All other uses are considered investigational. In accordance with US federal regulations an IDE and/or
IRB approval may be required.
The Magstim® Super Rapid² Plus¹ is considered an investigational device. Limited by Federal (or United States) Law to investigational use. In accordance with US federal regulations an IDE and/or IRB approval may be required.
