
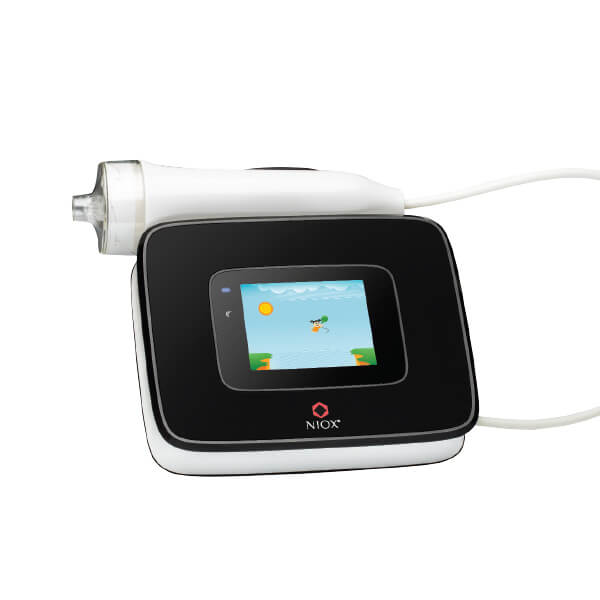
Accurately measure airway inflammation with NIOX VERO®
NIOX VERO is one of the most used FeNO testing devices worldwide.
Designed to help combat asthma
Asthma’s complexity and heterogeneity means that it’s not always easy to feel confident in diagnosis and management.2 With the prevalence of asthma increasing, poorly controlled asthma can have long term consequences for patients.
Improve patient outcomes with FeNO
FeNO testing is now recommended for asthma care in guidelines across the globe.6,7 Having an accurate FeNO testing device is essential in correctly diagnosing and managing asthma. FeNO values are well established and can help to optimise your treatment decision. The current ATS FeNO thresholds7 were derived from trials where NIOX technology was the most widely used device.8 Studies show that results from other FeNO testing devices are not comparable with NIOX and this suggests that NIOX may offer a more reliable reading when managing asthma according to ATS-validated FeNO cut off values.
Designed to give an accurate reading in only one measurement.
References
References: 1. Data on file. Circassia Ltd. October 2019 C-NIOX-0002. 2. Hanania NA et al. Measurement of fractional exhaled nitric oxide in real-world clinical practice alters asthma treatment decisions. Ann Allergy Asthma Immunol. 2018 120(4):414-418. 3. Sears MR. Lung function decline in asthma. Eur Respir J. 2007 30(3):411-3. 4. Bai TR et al. Severe exacerbations predict excess lung function decline in asthma. Eur Respir J. 2007 30(3):452-6. 5. O’Byrne PM et al. Severe exacerbations and decline in lung function in asthma. Am J Respir Crit Care Med. 2009 179(1):19-24. 6. National Institute for Health and Clinical Excellence (NICE). Asthma: diagnosis, monitoring and chronic asthma management. NICE guideline [NG80]. 2017. 7. Dweik RA, Boggs PB, Erzurum SC, et al; on behalf of the American Thoracic Society Committee on Interpretation of Exhaled Nitric Oxide Levels (FeNO) for Clinical Applications. An official ATS clinical practice guideline: interpretation of exhaled nitric oxide levels (FeNO) for clinical applications. Am J Respir Crit Care Med. 2011;184:602-615. 8. Data on file. Circassia Ltd. October 2019 C-NIOX-0001. 9. Huang T et al. Fractional exhaled nitric oxide measurement: Comparison between the Sunvou-CA2122 analyzer and the NIOX VERO analyzer. J Asthma. 2019 12:1-8. 10. Molino A et al. Comparison of three different exhaled nitric oxide analyzers in chronic respiratory disorders. J Breath Res. 2019 13(2):021002. 11. Saito et al. Comparison of fractional exhaled nitric oxide levels measured by different analyzers produced by different manufacturers. J Asthma. 2019 22:1-11. 12. Kapande KM et al. Comparative repeatability of two handheld fractional exhaled nitric oxide monitors. Pediatr Pulmonol. 2012 47(6):546-50. 13. Tsuburai T et al. Differences in fraction of exhaled nitric oxide values measured by two hand-helded analysers. Arerugi. 2017 66(3):204-208. 14. Alving K et al. Validation of a New Portable Exhaled Nitric Oxide Analyzer, NIOX VERO®: Randomized Studies in Asthma. Pulm Ther. 2017 3(1):207-218. 15. Syk J et al. Anti-inflammatory treatment of atopic asthma guided by exhaled nitric oxide: a randomized, controlled trial. J Allergy Clin Immunol Pract. 2013 1(6):639-48. 16. Petsky H. Exhaled nitric oxide in children with asthma. Respiratory care nurse’s perspective. Indian Pediatr. 2014;51(2):102-103.